Give The Name For Kmno4
What is Potassium Permanganate?
Potassium Permanganate (KMnO4) is an inorganic chemical compound. It is also known as Condy's crystals or permanganate of potash.
A High german-Dutch chemist Johann Rudolf Glauber was the get-go to discover the production of KMnO4 in the year 1659. This compound is h2o-soluble and consists of two ions: Permanganate ion and potassium ion. It is a nighttime majestic odourless solid in its physical state.
When potassium permanganate crystals are dissolved in water the solution formed is imperial. It is considered as a potent oxidizing amanuensis and does not produce toxic by products. It is usually prepared from other minerals such as manganese oxide.
Tabular array of Contents
- Structure of Potassium Permanganate
- Preparation of Potassium Permanganate
- Concrete Backdrop of Potassium Permanganate
- Chemical Properties of Potassium Permanganate
- Reactions of Potassium Permanganate
- Uses of Potassium Permanganate
- Effects on Wellness
- Solved Case
- Frequently Asked Questions – FAQs
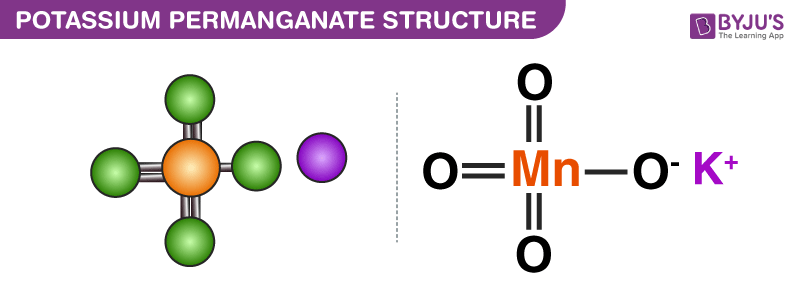
Structure of Potassium Permanganate (KMnO4)
The structure of potassium permanganate molecules is illustrated below. Notation that this chemical compound features an ionic bail between the potassium cation and the permanganate anion.
Training Of Potassium Permanganate – KMnO4
Potassium permanganate is commercially prepared by mixing solution of KOH and powdered manganese oxide, with oxidizing agents like potassium chlorate. The mixture is boiled evaporated and the residue is heated in iron pans until it has acquired a pasty consistency.
6KOH + 3MnOtwo + 6KClO3 → 3KtwoMnO4 + 6KCl + 3H2O
The potassium manganate (green) so formed is boiled with a large quantity of water and electric current of chlorine, CO2 and ozonized air is passed into the liquid until it is converted into permanganate. The MnO2 formed is removed continuously in society to foreclose its breaking down the permanganate.
6K2MnO4 + 3Cl2 → 6KMnO4(Potassium Permanganate) + 6KCl
The solution of KMnO4 is drawn off from any precipitate of MnOtwo full-bodied and crystallized. The crystals are centrifuged and dried.
Physical Backdrop Of Potassium Permanganate – KMnO4
- It is an odourless, regal to magenta crystalline solid.
- Information technology is soluble in h2o, acetone, acetic acid, methanol, and pyridine.
- It gets dissolved in ethanol and organic solvents.
- Potassium permanganate occurs in the form of monoclinic prisms, most opaque with a blue metallic lustre.
- It is odourless. An aqueous solution has a sweetish astringent gustatory modality. It is h2o-soluble and more soluble in boiling water.
Chemic Properties Of Potassium Permanganate
- Potassium permanganate is a very strong oxidizing agent and can, therefore, be used as an oxidant in a wide spectrum of chemic reactions.
- The oxidizing power of potassium permanganate can exist seen while performing a redox reaction with it, in which the dark purple solution turns colourless then into a brown solution.
- The higher up reaction tin can be performed in an acidic or a basic medium.
| KMnOiv | Potassium permanganate |
| Compound Proper name | Potassium manganate(7) |
| Molecular Weight/ Molar Mass of Potassium permanganate | 158.034 chiliad/mol |
| Density of Potassium permanganate | 2.703 chiliad/cm³ |
| Storage temperature of Potassium permanganate | Room temperature |
| Humid Point of Potassium permanganate | 100oC |
| Melting Point of Potassium permanganate | 240°C |
| Oxidation State | +7 |
Reactions Of Potassium Permanganate (KMnO4)
i. Thermal decomposition:
When solid potassium permanganate is heated information technology undergoes decomposition. The reaction is equally follows:
2KMnO4 → YardtwoMnO4 + MnO2(s) + O2
two. Reaction with acid:
When permanganate reacts with concentrated muriatic acid it produces chlorine. In a neutral solution, permanganate is reduced past three electrons to produce manganese dioxide, where the oxidation state of manganese is +four. Potassium permanganate reduces spontaneously in an alkaline solution and turns into green M2MnO4.
3. Outcome of Alkalies
On heating with alkalies, potassium permanganate changes into manganate and oxygen gas is evolved.
4KMnOiv + 4KOH → 4K2MnO4 + 2H2O + O2
iv. Oxidizing backdrop
KMnO4 acts as a very powerful oxidizing agent in acidic, neutral and element of group i media. The equations representing oxidation in these media are
In acidic medium
2KMnOiv + H2SO4→ K2SO4 + 2MnSOfour + 3HiiO + five[O] MnO4 – + 8H+ + 5e– → Mn2+ + 4H2O
In neutral or alkaline medium
2KMnO4 + HiiO → 2KOH + 2MnO2 + iii[O] MnO4 – + 2HiiO + 3e– → MnO2 + 4OH–
Uses Of Potassium Permanganate (KMnO4)
At that place are wide applications of KMnO4. Some of import uses of potassium permanganate have been discussed below:
- Potassium permanganate is used in qualitative analysis to determine the permanganate value
- KMnO 4 is also used as a regeneration chemical in well h2o treatment for the removal of hydrogen sulphide and iron
- This chemical compound is as well used equally a disinfectant to cure certain peel conditions like foot fungal infections, dermatitis
- Another of import application of potassium permanganate is in the handling of bacterial infections
- KMnO 4 is as well known to exist used in tanning leathers, printing fabrics
- This compound tin even be used equally a bleaching amanuensis, equally a pesticide, and as an clarified
- One of the virtually of import industrial applications of potassium permanganate is as an oxidizing agent in the chemical synthesis of many of import compounds.
Furnishings on Health
- In a concentrated class, potassium permanganate is an irritant to homo eyes and skin. Information technology can react with many reducing agents or organic material only it is inflammable.
- The antibacterial action of KMnO 4 is dependent on the oxidation of proteins of bacteria or tissues past this compound . It leaves a stain on pare or tissues. Since information technology acts past subversive oxidation procedure on all organic matter, its utilize is restricted for external purposes simply.
- Potassium permanganate acts every bit an antidote in barbiturates, chloral hydrate, and alkaloidal poisoning. A solution of 1:5000 of permanganate when used as a gastric wash, oxidizes toxicant and prevents their absorption.
- This chemical compound is normally stored in tightly closed containers. Potassium permanganate should be handled with intendance since an explosion may occur when information technology comes in contact with readily oxidizable substances.
Solved Example
Question:
Give the ionic equation for the oxidation of iodides with KMnOiv in acidic medium and in alkali metal medium.
Solution:
In acidic medium:
[MnOiv – + 8H+ + 5e– → Mntwo+ + 4H2O ] x 22I– →
I2 + 2e– ] x 5
———————————————————————–
10I– + 2MnO4 – + 16H+ → 2Mn2+ + 5I2 + 8H2O
———————————————————————–
In alkaline medium:
MnO4 – + 2HiiO + 3e– → MnO2 + 4OH– ] x 2
I– + 6OH– → IOiii – + 3H2O + 6e–
————————————————————————
2MnO4 – + I– + H2O → IO3 – + 2MnOtwo + 2OH–
————————————————————————
Frequently Asked Questions – FAQs
Write the formula for potassium permanganate
The chemical formula of potassium permanganate is KMnOiv.
Permanganate a good oxidizing agent. Why?
As the oxidation states of atoms increase the elements get more electronegative. Therefore, permanganate a good oxidizing agent.
List two uses of potassium permanganate.
2 uses of potassium permanganate are-
It is used to treat various skin diseases such as fungal infection in the foot
It is used equally an oxidizing agent in industries.
What is the colour of potassium permanganate?
Potassium permanganate'south physical land is an odourless solid, and they wait like crystals of nighttime imperial or statuary colour. When these crystals are dissolved in water, the solution becomes purple.
Why KMnO4 is a self indicator?
The solution under examination loses its pink colour once all the permanganate ions are used up in the reaction. It suggests the end of the reaction and therefore a self-indicator is called potassium permanganate, as it serves as an indicator apart from being ane of the reactants.
Learn more most the chemical behaviour and importance of KMnOfour with the expert faculties at BYJU'South.
Read more:
- Chemical compound formula
- Chemical formula
Give The Name For Kmno4,
Source: https://byjus.com/chemistry/kmno4/
Posted by: davissupostan.blogspot.com


0 Response to "Give The Name For Kmno4"
Post a Comment